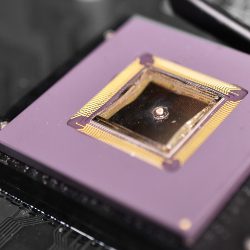



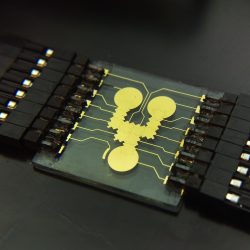







Microrespirometer
The microrespirometer project aims at developing a multi-sensor platform that can simultaneously measure key elements essential for cell metabolism, such as oxygen, glucose, and lactate. By miniaturizing the sensor design, the sensor platform can perform measurements with small quantity of biological samples in a small volume. We have so far focused on measurement techniques to capture rates of cellular oxygen consumption (OCR) using oxygen sensor and extracellular acidification (ECAR) using pH sensor since they are widely used as proxies for mitochondrial oxidative phosphorylation (OXPHOS) and glycolytic rate in cell metabolism studies.
Oxygen sensing is based on the principle of the Clark sensor as shown below. while pH sensing is based on potentiometric sensing using ISFETs.

The microphotograph below shows the 1st generation multi-sensor platform that incorporated an O2 sensor and a pH sensor in the same platform. The other two sensors in the package can be used as a glucose and a lactate sensor, respectively.

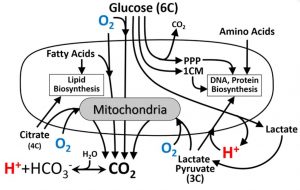
Understanding the fundamentals of cell metabolism is important to designing the multi-sensor platform. As illustrated below, oxygen is consumed by respiring mitochondria in cells to support oxidative metabolism of multiple substrates. Hydrogen ions (H+) are exported from cells with lactate but can also be generated from CO2 in the aqueous buffer or co-transported into cells along with pyruvate or lactate. The six carbons of glucose can be released as CO2 during oxidative metabolism, as two lactate molecules following glycolysis, or utilized in the biosynthetic pentose phosphate pathway (PPP) and one-carbon metabolism (1CM) pathway.
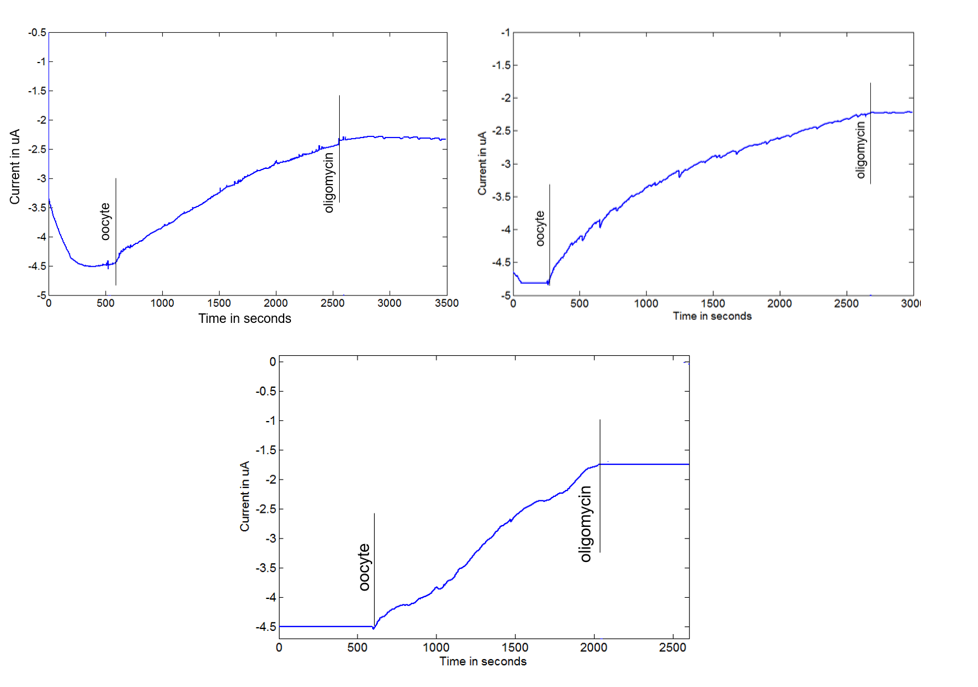
The idea of capturing rates of cellular oxygen consumption (OCR) and extracellular acidification (ECAR) is to capture the steady change in oxygen concentration and pH over a period of time when the target biological samples are present in the measurement chamber. The following graphs show the changes (reduction) in oxygen concentration over time obtained by the multi-sensor platform after a bovine oocyte was placed in the measurement chamber (marked as “oocyte” in the graphs). The slope of the oxygen concentration curve provides the rate of cellular OCR for the given oocyte. The obtained cellular OCR was further verified when oligomycin was introduced in the measurement chamber (marked as “oligomycin in the graphs) to block mitochondrial oxidative phosphorylation (OXPHOS), forcing the oocytes to rely on glycolysis. This resulted in a dramatic reduction in oxygen consumption by the oocytes shown as an unchanging oxygen concentration over time. This clear demonstration of shifts in cell metabolism during development and in response to OXPHOS inhibition as a model system for monitoring metabolic plasticity in very small biological samples has a wide potential utility for analyzing small biological samples such as single cells and tumor biopsies for immunology and cancer research applications.
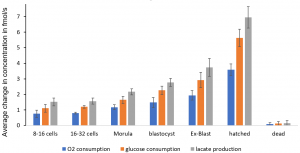
Monitoring multiple analytes in a single sensor platform can enable detailed analysis of cell development over time, providing unique insight into healthy vs. disease cells. The following graph shows the results of measuring oxygen and glucose consumptions, and lactate production of embryos from 8-16 cells to hatched blastocysts.
The latest generation of the microrespirometer incorporates microfluidic support to allow easy and more seamless implementation of complex experiment protocols. The diagram below illustrates the latest generation of the multi-sensor platform design, where (A) shows the latest design of the multi-sensor chip; (B) shows a microchamber design with a microfluidic inlet (left) and an outlet (right) on the top; and (C) shows an actual sensor chip with the microchamber mounted on it.

The latest generation of the multi-sensor platform, together with its microfluidic support system, allows integration of additional components. such as syringe pumps and incubators. The photograph below shows a setup of the measurement system incorporating different components needed to enable a complex experiment protocol.

The high level of system integration has allowed us to expand the applications of the multi-sensor platform to other areas of interests in cell metabolism, such as real-time measurement of oxygen consumption rate (OCR) and ROS production using an oxygen sensor and a hydrogen peroxide sensor. The graph below shows the changes of hydrogen peroxide production rate (HPR) and OCR of mitochondria during the transitions from the basal state to the LEAK state (“LEAK” respiration in the absence of ADP), and to the OXPHOS-linked state. OCR did not change significantly from basal to LEAK state. However, it increased 167.94% from 2944.96 pmol∙s-1∙mg-1 to 7890.51 pmol∙s-1∙mg-1 from the LEAK state to the OXPHOS state (A and C), consistent with high respiratory control of cardiac mitochondria by ADP. In contrast, the HPR increased over 350% from the basal state to the LEAK state when mitochondria were energized by substrates (LEAK), then decreased 73.29% from 201.3 pmol∙s-1∙mg-1 to 53.78 pmol∙s-1∙mg-1 (P < 0.01) following the transition to the OXPHOS state (A and B). This trend is consistent with the strong influence of mitochondrial membrane potential on ROS production by the respiratory chain, which is highest during the LEAK state and dissipated by the activity of ATP synthase in the OXPHOS state. These results illustrate that the multi-sensor platform has the sufficient sensitivities and linearities necessary for both H2O2 and O2 to enable advances in instrumentation technology that can improve our understanding of cellular bioenergetics in health and disease.

Walter Scott, Jr. College of Engineering
www.engr.colostate.edu
700 Meridian Ave
1301 Campus Delivery
Fort Collins, CO 80523-1301
(970) 491-3366
School of Biomedical Engineering
www.engr.colostate.edu/sbme
700 Meridian Ave
1376 Campus Delivery
Fort Collins, CO 80523-1376
(970) 491-7157
Walter Scott, Jr. College of Engineering
www.engr.colostate.edu
700 Meridian Ave
1301 Campus Delivery
Fort Collins, CO 80523-1301
(970) 491-3366
School of Biomedical Engineering
www.engr.colostate.edu/sbme
700 Meridian Ave
1376 Campus Delivery
Fort Collins, CO 80523-1376
(970) 491-7157

